Introduction To CNG Conversion For Gasoline Engine Vehicles
A Little History About The Use Of Natural Gas In Automotive
Humans have been using natural gas for thousands of years, in fact, it is believed that the Chinese already used it in 500 BC. Boil and purify water Natural gas was used to power the streetlights in 1785, and was the main source of light during the 19th century. The invention of the electric bulb in 1879 forced natural gas companies to seek other uses for the resource and, as a result, natural gas became a very popular source of energy.
Natural gas vehicles have been documented as early as the 1930s, although it is not clear exactly when or who invented them. The first Natural Gas vehicles operated with uncompressed gas and were called Gas Bag vehicles. These vehicles were used as a necessary way to combat the gasoline shortage during the First World War, and even more during World War II.
The first Natural Gas cars had a wood, or metal structure mounted on the roof and attached to the bumpers, with a huge and unmanageable balloon for a gas tank. These first vehicles attracted a lot of attention, and clearly had less than ideal aerodynamics. CNG tanks are still notoriously large, occupying approximately twice as much space as gasoline tanks for the equivalent amount of fuel. This can be discouraging, but we have come a long way and we continue to improve.
Although it is commonly called "transformation of a car to CNG" the installation in a vehicle is done maintaining the fuel system of gasoline, adding the compressed natural gas system, so that it is always possible to work alternately with one or another fuel (gasoline and compressed natural gas, for this reason they are also called "Bi-Fuel")
The installation of the entire system necessary to consume compressed natural gas as fuel in our car is simple, since the amount of components installed in the car is low, so the chances of breakdowns or malfunction are rare.
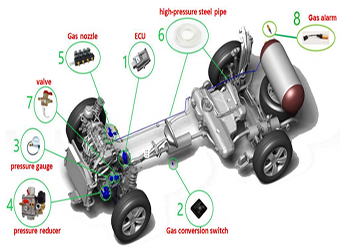
CNG technical data
CNG Heating Power : The CNG's Heating Power is around 2063 Kcal / liter, while Gasoline is around 8005 Kcal / liter. Which explains the loss of power in carburetion engines and the increase in consumption in the injection engines.
Storage Pressure : The Storage Pressure is at 200 bar, pressure at which it is kept compressed and which is approximately the pressure a submarine supports at 2000 meters. depth
Octano Index : The Octane Index for CNG is around 130 octane.
Density : Density around 0.158 kg / liter
Storage Temperature : The storage temperature is less than 0 degrees, which together with the necessary pressure results in a large amount of energy stored in a small volume.
Environmental factors to consider
NOx particulate emissions : In tests conducted by the Argonne National Institute, dependent on the Department of Energy of the United States Government with different models of vehicles and their versions CNG, Diesel and Gasoline results showed an emission of particles that they reduced emissions of diesel models by 95%. As well as a NOx emission reduction of 49%
CO2 emissions :In the same experience it was possible to verify that CO2 emissions were reduced by 20.8% with respect to the established limit and between 17 and 34% on gasoline emissions.
 Refer to:https://www.glpautogas.info/en/cng-conversion.html
Refer to:https://www.glpautogas.info/en/cng-conversion.html
The pictures and articles are from the internet. If there is any infringement, please contact us to delete them.
prev£ºnothing
Popular articles
-

How the CNG Automotive S
Compressed natural gas (CNG) automotive systems
-

What Is CNG Pressure Red
The pressure reducer of natural gas vehicle is
-
Advantages Of CNG Gas V
Compressed natural gas vehicles are vehicles th
-

Reasons For High Gas Con
1. Original vehicle condition A. The tec
-

Differences Between Sing
Characteristics of Gas Single Point Device
-

How To Improve The Power
1. Install ignition advance angle What i
-

Advantages And Principle
LPG and CNG are two mainstream alternati
-

How The CNG Gas Vehicle
If you want to know ¨C how does the CNG conversi






Latest comments
0piece comment
no comments, welcome to comment¡£